The European Union is poised to tighten rules on the export of Covid-19 vaccines in a move that risks fueling a global protectionist battle between countries vying for the life-saving shots.
As EU member states grapple with shortfalls in supplies from drugmakers including AstraZeneca Plc, the European Commission will on Friday require companies seeking to ship the jabs outside the bloc to request authorization. That will involve advance notification of the shipment amounts and destinations, EU officials in Brussels said.
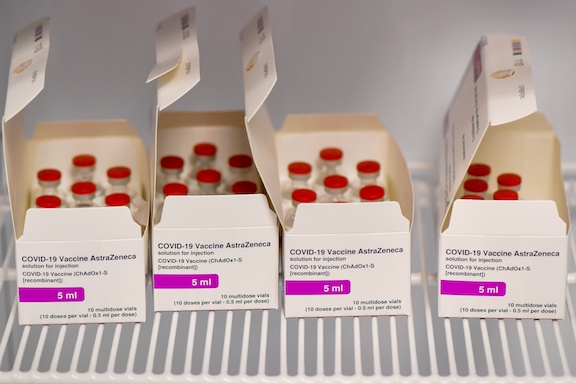
That will allow the bloc to refuse the license to export if a set of pre-defined criteria hasn’t been met, the officials said. The main condition will be that companies have delivered a sufficient number of dosages to the EU, as defined in existing purchase agreements.
The dramatic move, which in effect sets a “Europe first” system for vaccine deliveries, would mark a step-up in the EU’s struggle to reignite a campaign that got off to a sluggish start. The situation worsened after Astra said that production of shots will be slower than anticipated. The EU responded by demanding that Astra dip into U.K. supplies to make up the shortage.
In addition to the commission proposal, Friday could also see the European Medicines Agency approve the Astra shot for use. That would bring to three the number of vaccines authorized in the bloc.
Governments across the continent are under pressure amid disruption to the rollout of doses. On Thursday, Belgian health authorities inspected the factory at the heart of the Astra controversy, according to a person familiar with the situation. The Associated Press reported the commission requested the review as it’s unhappy with the explanations for the delay.
Astra shares fell for a second day, and were trading down 1.4% as of 15:30 p.m. in London.
Meanwhile, Belgium notified a draft law to the EU’s executive arm, seeking permission to limit the exports of essential medicines and active ingredients, according to people familiar with the matter.
That request cites the bloc’s urgency procedures, which allow crisis measures for the protection of human health, the people said. It’s currently being assessed by the commission, the people said.
The EU’s planned vaccines export-licensing system would mirror a European arrangement put in place for part of last year for personal-protective equipment such as masks, gloves and gowns to fight the coronavirus. While EU member-country authorities would normally approve requests within 24 hours, a key criterion will be whether the bloc’s advance purchase agreements for vaccines are being respected.
The commission has reached deals with vaccine developers for a total 2.3 billion doses for the bloc’s 27 countries. Of those, Astra accounts for 400 million doses.
The new system for vaccines will last until the end of March, with the possibility for an extension. EU officials have said the main goal is to get more transparency about the market situation.