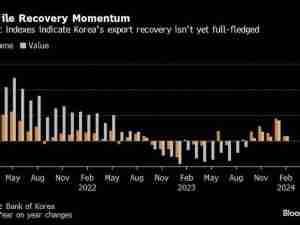
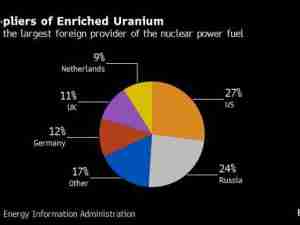
The European Union said it has approved a further 23 requests to ship Covid-19 vaccines to other parts of the world under the bloc’s new export-licensing regime, bringing the total to 27.
The disclosure on Wednesday by the European Commission, the EU’s executive arm, follows its announcement last week that it had endorsed four vaccine shipments from the bloc—two to Canada and one each to Japan and the U.K.
So far, the EU has approved all requests to export Covid-19 vaccines under the oversight system, according to the commission. It said the latest authorizations cover shipments to countries including Australia, China, Mexico, New Zealand, Oman and Saudi Arabia.

“The commission plans to communicate regularly on the implementation of this measure,” trade spokesperson Miriam Garcia Ferrer said by phone in Brussels.
The 27-nation EU established the export-authorization arrangement on Jan. 30 amid political calls in the bloc to ensure adequate domestic supplies and accelerate a relatively slow pace of inoculations.
Under the system, due to last until the end of March, drug companies must notify EU national authorities in advance of the amounts and destinations of any vaccine shipments to other parts of the world.
While the requests are supposed to get approved as a matter of course and quickly, a key criterion is whether advance purchase agreements reached by the EU for vaccines are being respected. That opens the door to possible rejections.
The EU has struck accords with various companies for a total of around 2.3 billion vaccine doses. So far, the bloc has given the regulatory go-ahead to shots developed by Pfizer Inc.-BioNTech SE, Moderna Inc. and AstraZeneca Plc.
“We do not intend to restrict companies that are honoring their contracts with the European Union,” commission President Ursula von der Leyen said on Wednesday in Brussels about the export-licensing system. “Europe is always ready to help, but we insist on our fair share.”